Atomic Layer Deposition (ALD)
A lot of the discussion in this tutorial has emphasized the importance of transport in determining how CVD reactors work. What if you are a chemist and hate differential equations? Or just would like very precise control over the growth of your film? An alternative approach to growing films is to make the surface chemistry control everything about the growth process. This approach is typically known as atomic layer deposition, or ALD. We expose the surface to one precursor that makes a part of the film, then remove that from the chamber and add a second precursor that makes the rest. By repeating the process, we grow films one atomic (or molecular) layer at a time. As you know if you've read the discussions of transport elsewhere in the tutorial, filling and emptying chambers takes substantial fractions of a second even at relatively low pressures, so making millions of atomic layers is painfully time-consuming. So ALD is particularly helpful for very thin films, where you can count the number of atomic layers on your fingers: for example, the gate dielectrics in incredibly tiny modern transistors. It's rather painful when you need a film whose thickness is measured in microns or millimeters.
The early work on ALD was done by Tuomo Suntola in Finland, and was targeted to thin films for flat panel displays. The technique was mostly ignored until the 1990's, but thereafter research expanded rapidly. This expansion coincided with, and was partly driven by, the requirement for silicon CMOS transistor gate oxides to achieve higher dielectric constants than could be provided by silicon dioxide or silicon oxynitrides. Since such gate oxides are just a handful of nanometers thick, growing them one atomic layer at a time is not a problem, with growth time happily traded off for process control. ALD is now widely used in semiconductor processing when it is economically feasible, and is also applied in other fields, wherever thick films are not needed and thickness control, or very good conformality, are.
Suntola's initial investigations looked at both separating reactants in time and in space. That is, one approach is sequential: you fill a reactor with one precursor, let it saturate the surface, then purge and fill it with the other precursor, repeating this cycle in time to grow the layers. This typically uses either lateral transport (like the tube reactors described in the tutorial) or vertical transport as seen in showerhead reactors. An alternative approach is to separate the reactants in space by using laminar streams with purge gases between them, and then move the substrate through the streams. This second approach is conceptually similar to the APCVD reactor in the tutorial, and indeed some processes can be performed at atmospheric pressure, simplifying the overall equipment problem. The time-cycling approach dominated applications in the semiconductor industry and elsewhere for many years, but the spatial approach experienced a resurgence in the mid-2000's. Spatial ALD is particularly well suited for roll-to-roll continuous processing, where a thin flexible substrate passes through zones with different gas ambients, allowing reactions to proceed in time sequence from the viewpoint of the substrate surface.
An Example: Aluminum Oxide from TMA and Water
Let's take a quick look at a system that people have done a lot of work on: the deposition of aluminum oxide, Al2O3, from trimethylaluminum (TMA) and water.
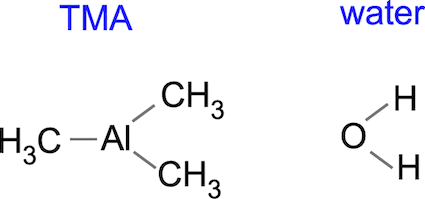
The basic idea is to expose a hydroxyl-terminated surface to TMA to form a layer of adsorbed aluminum connected to the surface oxygen and terminated by methyl groups. We then expose the methyl groups to water, ideally producing volatile methane CH4 and returning the surface to -OH termination. We grow one monolayer of aluminum oxide at a time. A simplistic view of the process is depicted below. Let's imagine we start with a hydroxyl-terminated silicon surface (which might be the case if we're processing a silicon wafer). When we add TMA, we hope that the methyl groups will react with the hydroxyls to end up with a methyl-terminated -Al-CH3 surface.
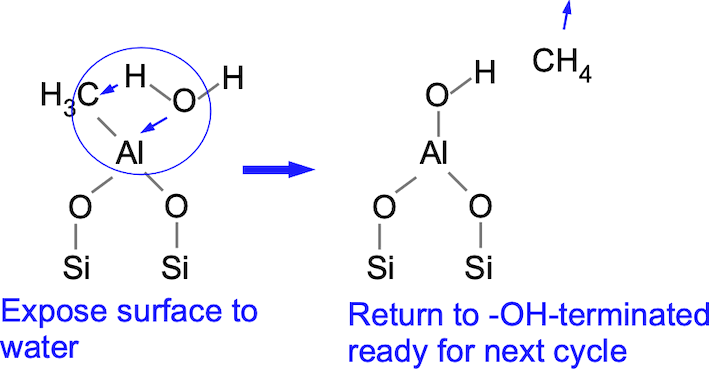
We then remove the TMA and expose the surface to water. Ideally, the water will react with the aluminum and extract the methyl, leaving behind a hydroxyl-terminated surface like what we started with.
A more nuanced view of what's going on is provided in recent work using density-functional theory, a now-popular approach to simplifying the computations involved in computing the energy of molecular configurations ("Density Functional Theory (DFT)-enhanced computational fluid dynamics modeling of substrate movement and chemical deposition process in spatial atomic layer deposition", D. Pan, Chem Engr Science 234 p. 116447 (2021)). We will briefly review some of Pan's results below.
In this work, the first step is the formation of a bond between the Al of an incident TMA molecular and an (-OH) group on the surface, forming a trivalent oxygen atom. (Here we are again assuming we start with a silicon substrate.) The hydrogen is grabbed by one of the methyl groups (becoming temporarily divalent); a methane molecule results, departing into the gas phase and leaving an adsorbed dimethyl-aluminum group. Then, assuming a neighboring -OH is available, the Al bends to bond to it, once again resulting in an unstable trivalent O. However, here it's getting tough for the methyl to bend far enough to grab the extra hydrogen — in chemistry parlance, this reaction is often sterically hindered and may proceed slowly. If it goes to completion, another methane is released and we're left with a nice mono-methyl-terminated surface. But it's reasonable to guess that real surfaces might be a mixture of mono- and di-methyl configurations.

Once we've reached whatever surface results from the TMA exposure, we presume that the TMA is removed from the environment and water vapor is added. (Actually doing this is an important practical issue, using up a lot of process time or space.) Pan et. al. first look at a singly-bonded Al-O-(substrate). The incoming water molecule typically attaches to the aluminum, and then has one of its hydrogen atoms stolen by the neighboring methane, which then is released to the gas ambient. Note that this reaction can also be sterically hindered as the methanes get in each other's way.
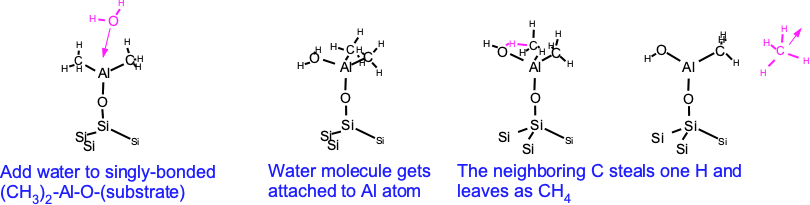
A second water molecule can then stick itself onto the aluminum, again providing the final terminating H to the neighboring methyl group, which is able to leave as methane. A similar reaction occurs when a singly-terminated Al-CH3 is present. The result is a hydroxyl-terminated surface similar to what we started with, ready for another purge and TMA exposure step.

A similar reaction takes place on the double-bonded Al regions.
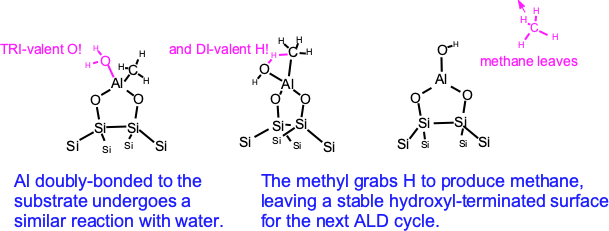
In both cases we return to a surface that is terminated with hydroxyls — but NOT with exactly the same geometry! ALD is not just sticking simple layers on top of one another; real processes can be complicated.
NOTE that it's very difficult to experimentally confirm that the modeled configurations are what really happens. We should view Pan et. al.'s results, as shown above, as a plausible view of what's going on at an atomic level, not necessarily right in every detail but providing guidance about what's happening and what can go wrong.
Further reading:
Brief Overview of Microsystems and Nanoelectronics, K. Elers, VTT Technical Research Centre of Finland, Seminar Presentation at UC Berkeley, January 30, 2010; https://microlab.berkeley.edu/text/seminars/slides/VTT_ALD.pdf
Atomic Layer Deposition in Semiconductor Manufacturing, J. Trelles, Washington State University, November 7, 2011; https://faculty.uml.edu//juan_pablo_trelles/publications/documents/trelles_ald_for_semiconductor_manufacturing_wsu.pdf
Spatial Atomic Layer Deposition, D. Munoz-Rojas, V. Nguyen, C. de la Huerta, C. Jimenez and D. Bellet, Chapter in Chemical Vapor Deposition for Nanotechnology, Intech Open https://www.intechopen.com/chapters/64743
Return to Tutorial Table of Contents
Book version of the CVD Tutorial
