Silicon Nitride: Properties and Applications
"Bulk" silicon nitride, Si3N4, is a hard, dense, refractory material. Its structure is quite different from that of silicon dioxide: instead of flexible, adjustable Si-O-Si bridge bonds, the Si-N-Si structure is rendered rigid by the necessity of nitrogen forming three rather than two bonds. The nitrogen atoms are arranged roughly tetrahedrally around the silicon, as in silicon dioxide:
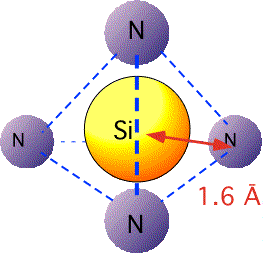
However, with respect to the nitrogen atoms, the silicon neighbors are arranged at the vertices of a planar triangle:
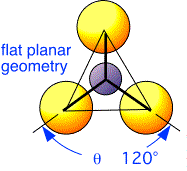
The bonding around the nitrogen atoms is therefore somewhat sp2-like: that is, three hybrids of s, px and py form three bonds to silicon atoms, with the pz orbitals (sticking out of the plane) non-bonding.
The detailed structure of the crystalline forms of silicon nitride is known but is very complex.
refs: "The Crystal Structures of Alpha and Beta Silicon and Germanium Nitrides" S. Wild, P. Grieveson and K. Jack in Special Ceramics 5, ed. P. Popper, British Ceramic Research Association, 1972; "Structure and Chemical Composition of Silicon Nitride" chapter 4 of Silicon Nitride in Electronics, Belyi et. al., T Elsevier 1986; “ The electronic properties of silicon nitride" J. Robertson Philosophical Magazine B44 215 (1981)
CVD silicon nitride is generally amorphous, but the material is much more constrained in structure than the oxide. As a consequence, nitride is harder, has higher stress levels, and cracks more readily.
The dense structure of silicon nitride does not provide the open channels found in oxide structures; thus, nitride is widely employed in electronics as a barrier material. Even hydrogen diffuses slowly in a densified nitride film, and other small positive ions (Na+ or K+) are effectively blocked by thin nitride layers. Since oxygen diffuses very slowly through nitride, deposited nitride can prevent oxidation of underlying silicon: this property is exploited in the old local-oxidation -of-silicon (LOCOS) transistor isolation. Nitride layers are also employed as etch stop layers both for wet etching and plasma etching.
Deposited nitrides almost always contain hydrogen, typically much more than in the comparable oxide films. The source of the hydrogen is the silane precursor and possibly also the ammonia oxidant employed in most deposition schemes, but the presence of hydrogen in the film is a consequence of the nitride structure. It is very difficult for the atoms in an amorphous but constrained film like silicon nitride to all occupy positions allowing the valence of each silicon and nitrogen atom to be filled: that is, a lot of broken bonds are present. These bonds are readily occupied by hydrogen atoms. Thus, conventional plasma nitrides can have as much as 20 atomic % hydrogen, bonded both to the Si and N atoms; thermal nitrides still have several % hydrogen even after high-temperature anneals. Note that the group Si-NH-Si is isoelectronic to Si-O-Si; the addition of NH groups adds flexibility to the nitride lattice.
The amount of hydrogen and the bonding (Si-H or N-H) can be measured readily by infrared spectroscopy, and are important in characterizing the properties of plasma nitrides. The stoichiometry of nitride films also varies widely, especially in plasma deposition, so that refractive index can vary from about 1.8 to 2.2 and is another useful control parameter for nitride deposition. The type of bonding (N-H vs. Si-H) influences the UV transparency of the films.
In plasma deposition, mixtures of oxygen and nitrogen with silicon -- silicon oxynitrides -- can be prepared by introducing small amounts of oxidant. The properties of the films can be varied fairly continuously from those of the pure oxide to the "pure" (hydrogenated) nitride.
Let's look at some important properties of pure Si3N4:
density 3 - 3.3 gm/cm3 electrical conductivity varies widely breakdown field typically a few 10^6 V/cm thermal conductivity 0.15 W/cm K (bulk) thermal diffusivity 0.07 cm2/sec (bulk) coefficient of thermal expansion 3 ppm/ K [note Si thermal exp 2.3 ppm/K] dielectric constant 6-8 [depends on stoichiometry]
Return to Tutorial Table of Contents
Book version of the CVD Tutorial
