Silane / Oxygen Thermal CVD of Silicon Dioxide
Silane and Oxygen: The Basics
Silane is more properly monosilane, SiH4, a gas at room temperature. Silane is stable in storage even at high pressures and when exposed to shocks, but when mixed with oxygen will burn or explode. It must be handled with care: typically silane tanks are placed in gas cabinets specifically designed for explosive gases, with dedicated nitrogen supplies for purging. Silane can react with traces of moisture in gas lines to form powder which leads to particles and can clog valves and mass flow controllers; thus, carefully cleaned and purged stainless-steel plumbing is essential. Silane leaks can be exceedingly hazardous: it is possible for silane gas to collect in the atmosphere and then explode violently when disturbed (such as when a gas cabinet door is opened to search for the leak!). Silane is also moderately toxic, though explosion hazards are usually more severe. It is thus a good practice to employ trace monitors (toxic monitoring) in a silane facility. The common dopant chemicals, diborane B2H6 and phosphine PH3, are extremely toxic and absolutely require toxic monitoring. Note that even with toxic monitoring in place, care is necessary. It is a sobering exercise to calculate the diffusion length (or equivalently the Peclet number) for a typical room environment with a toxic gas leak: in a 5 minute "air change time" the diffusion length is only about 12-15 cm. Rooms are not necessarily well-mixed and there is no guarantee that the leak will reach poorly-chosen toxic monitor sense points before reaching an operator's face.
Now that the reader is properly frightened, we can say that despite the dangers, silane is widely used in semiconductor manufacturing, and with proper precautions can be handled safely and reliably.
Deposition
The basic overall reaction for the deposition of silicon dioxide requires the removal of the hydrogen atoms and addition of two oxygens:
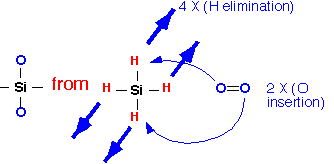
When oxygen is present in excess, water is the main byproduct (APCVD), whereas in low-oxygen conditions, hydrogen will be produced (LPCVD). Because the heat of formation of silane is small and that of silicon dioxide is large, this process is very exothermic:

The reaction proceeds through a complex series of reactive gas-phase intermediates; an example, in which water produced by the burning of other silane molecules attacks a silene radical, is:
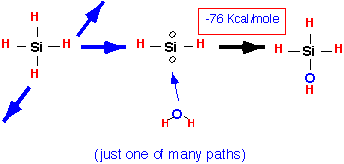
Note how one of the intermediate species in this reaction is the biradical SiH2; in general, many radicals are produced and branching chains are certainly possible, which is why silane/oxygen mixtures can explode. At the surface, these various reactive compounds tend to stick readily, giving high RSC and mediocre conformality. Once they are in place on the surface, additional "condensation" reactions must occur to remove the excess hydrogen and form the bridge bonds of the oxide film, with water or hydrogen being produced:
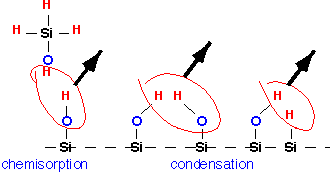
Deposition requires temperatures in excess of about 250 °C, but at higher temperatures there is little effect of temperature on deposition rate:
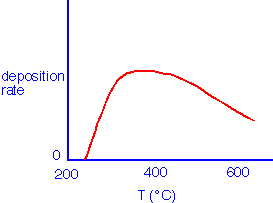
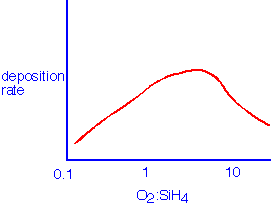
Semiconductor applications usually require temperatures in excess of 350 °C to obtain reasonable film density and purity. Absolute deposition rates of 100 nm/minute are achievable in single-wafer (showerhead) LPCVD, and local deposition rates of 500 nm/minute in injector APCVD. The rate also depends on the initial gas composition, with a maximum in rate being observed at moderate excess of oxygen:
Consequences of Chemistry
Conformality and gap fill: Deposition is due to reactive species with relatively high sticking coefficients (RSC about 0.3). Conformality is much better than e.g. sputtering or evaporation, but the ability to cover or fill high-aspect-ratio features (>1:1) is limited.
Rough film, dirty chamber: Reactions in the gas phase are rapid, and depending on gas flow and mixture can lead to growth of larger nuclei. If these are incorporated into the film the surface is roughened; downstream they grow to dust which is deposited on chamber walls and accumulates in the exhaust.
Films contain hydrogen: In moderately oxidizing conditions (e.g. tube LPCVD) Si-H will be present in the film. When copious oxygen is present during growth (APCVD) the film contains Si-OH. Excessive H in the films can lead to various problems; however, these problems are generally much more serious in TEOS films and will be discussed in connection with that precursor.
Return to Tutorial Table of Contents
Book version of the CVD Tutorial
