Examples of Real Precursors
Let's look at examples of precursors that are often used for films important in microelectronics:
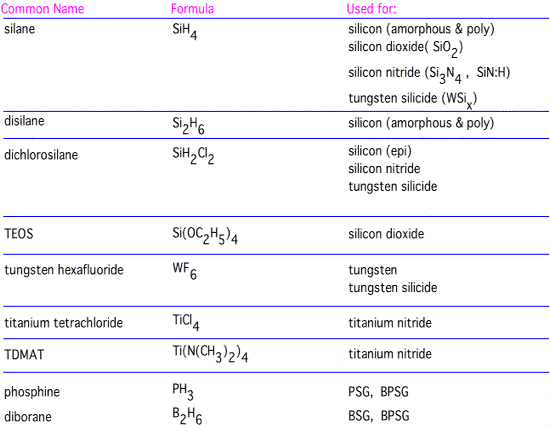
As we've discussed previously, each of these is a fairly small molecule, with saturated, non-polar bonds, to ensure good volatility. As we'll review in some more detail in the "films" section, differing precursors have different characteristics and do not produce identical films.
Let’s take a detailed look at a couple of the resulting chemical systems.
Energies and Transition States: Reactions and Species Involved in the Creation of Silicon Nitride
In the diagram below we summarize some calculations performed by Korkin and coworkers. They estimated the enthalpy of various gas-phase reactions that might be involved in the formation of silicon nitride from several precursors (dichlorosilane is shown here) mixed with ammonia, using a mixture of "first principles" calculations and empirical verification.
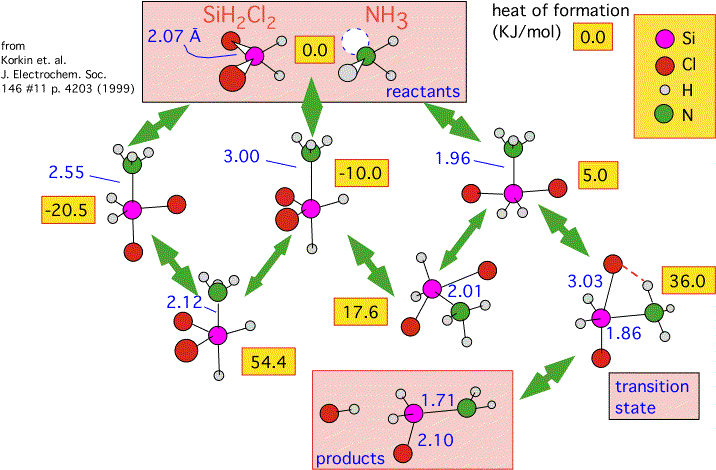
Recall that ammonia has a lone pair of electrons at the apex of the pyramidal molecule. Ammonia and dichlorosilane readily form an adduct: a weak compound in which the lone pair fills "empty" orbitals around the silicon atom. (Silicon, unlike carbon, has a fairly easy time expanding its "coordination sphere" -- that is, allowing more than 4 neighboring atoms.) The diagram shows that there are three different geometries in which the adduct can be formed, and that they can interconvert by passing through alternative conformations with fairly large activation energies: 54 and 18 KJ/mole. Note that the actual transition state to the final products (HCl and H2SiClNH2) is only reachable from the least energetically favorable of the metastable intermediates (+5 KJ/mole relative to the reactants).
We see how important geometry is: intermediates with exactly the same composition but different conformations have wildly differing energies. We can also see how complex the path can be to producing products, with a whole series of intermediates quite a bit more stable than the one that finally leads to the transition state. This system is a good illustration of why one ought to be suspicious of overly simplified kinetic arguments: the concentration of the reactive intermediate H2SiClNH2, itself an unstable and complex species, is determined by a complex quasi-equilibrium amongst various conformations of the adduct.
Si-O-H Molecules and Radicals
As a final example, we show some similar calculations of the heat of formation of various molecules likely to be involved in the formation of silicon dioxide from silane or organosilanes.
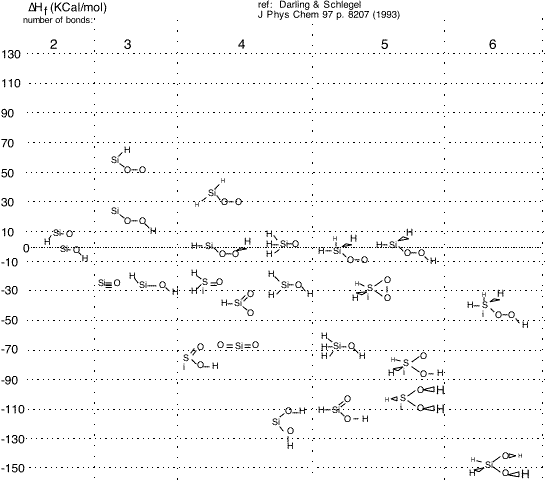
We see that various possible species are separated in energy by over 200 KJ/mole. From example [2] above we can guess that every one of these species also has a complex set of geometric isomers which may provide paths for conversions and reactions (if I followed the reference correctly, the states shown here are the lowest-energy conformation for each composition and bonding).
The heat of formation of a molecule, particularly a radical or metastable species, is not always easy to guess from the sort of simplistic bond energy addition procedure we discussed previously: for example, H3Si-O-O and H2-Si-O-O-H have about the same energy, even though the simplistic Si-H bond energy is much smaller than that of the O-H bond. Very general trends -- for example, triradical HSi-O-O is a very high-energy, improbable species -- are predicted by staring at the bonds, but to achieve any useful accuracy serious numerical resources are unavoidable.
The Last Word
If you read through all this, you'll probably agree that chemistry is a big, complex, nasty and fascinating field which we have not begun to explore in detail. However, you should have enough terminology and concepts at this point to proceed through the remainder of the tutorial, and to begin reading other sites, books, and technical papers without being completely lost.
Return to Tutorial Table of Contents
Book version of the CVD Tutorial
